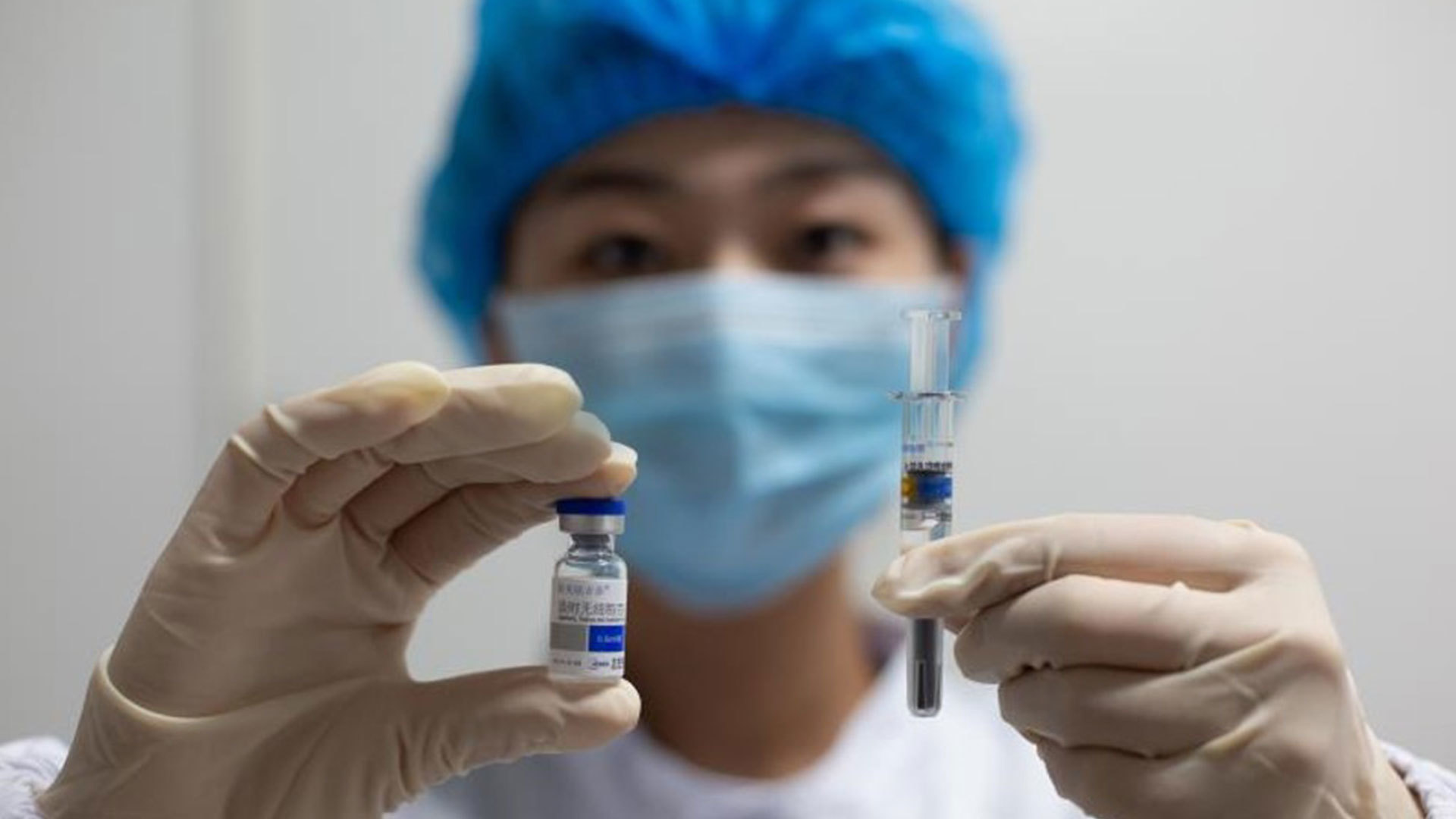
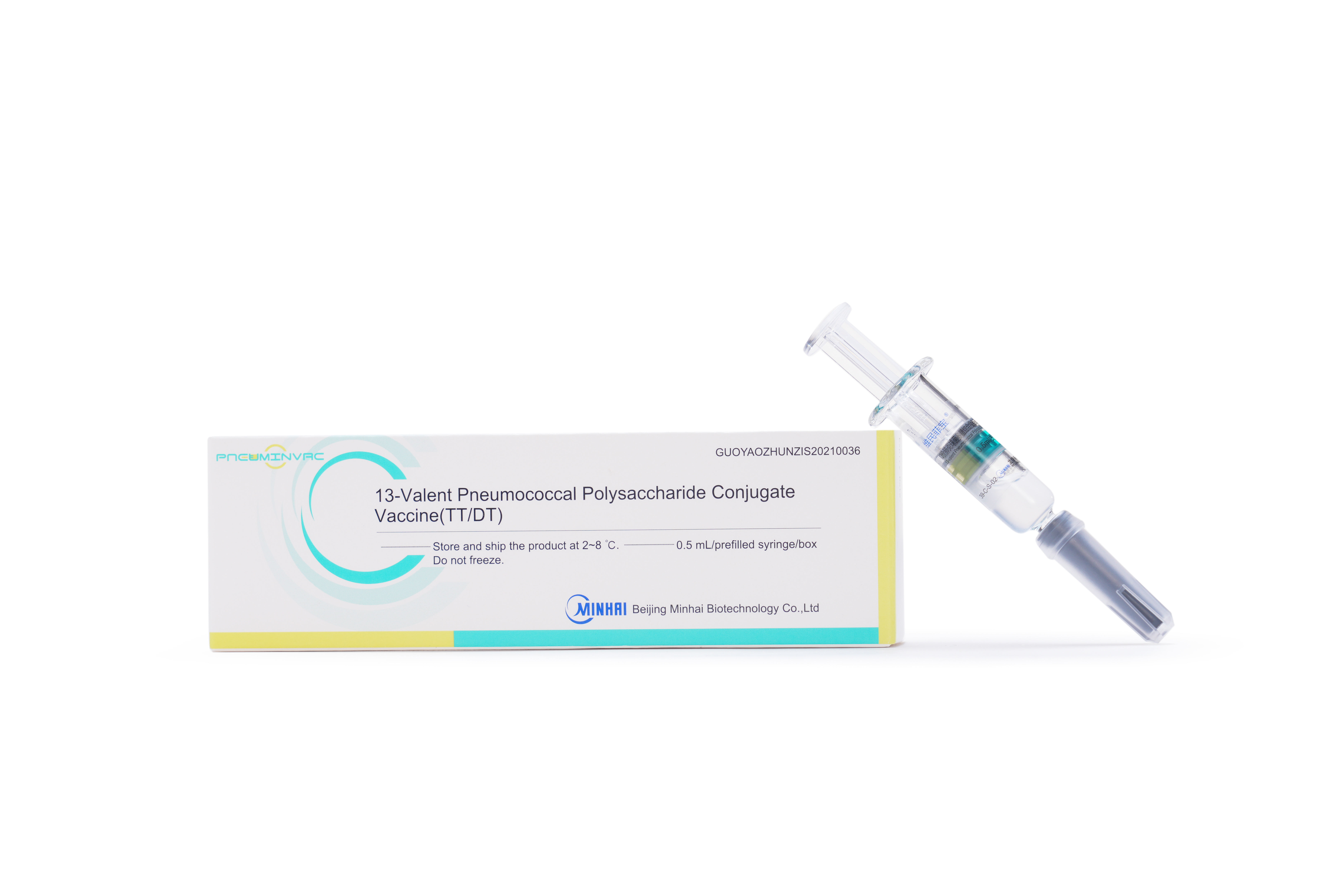
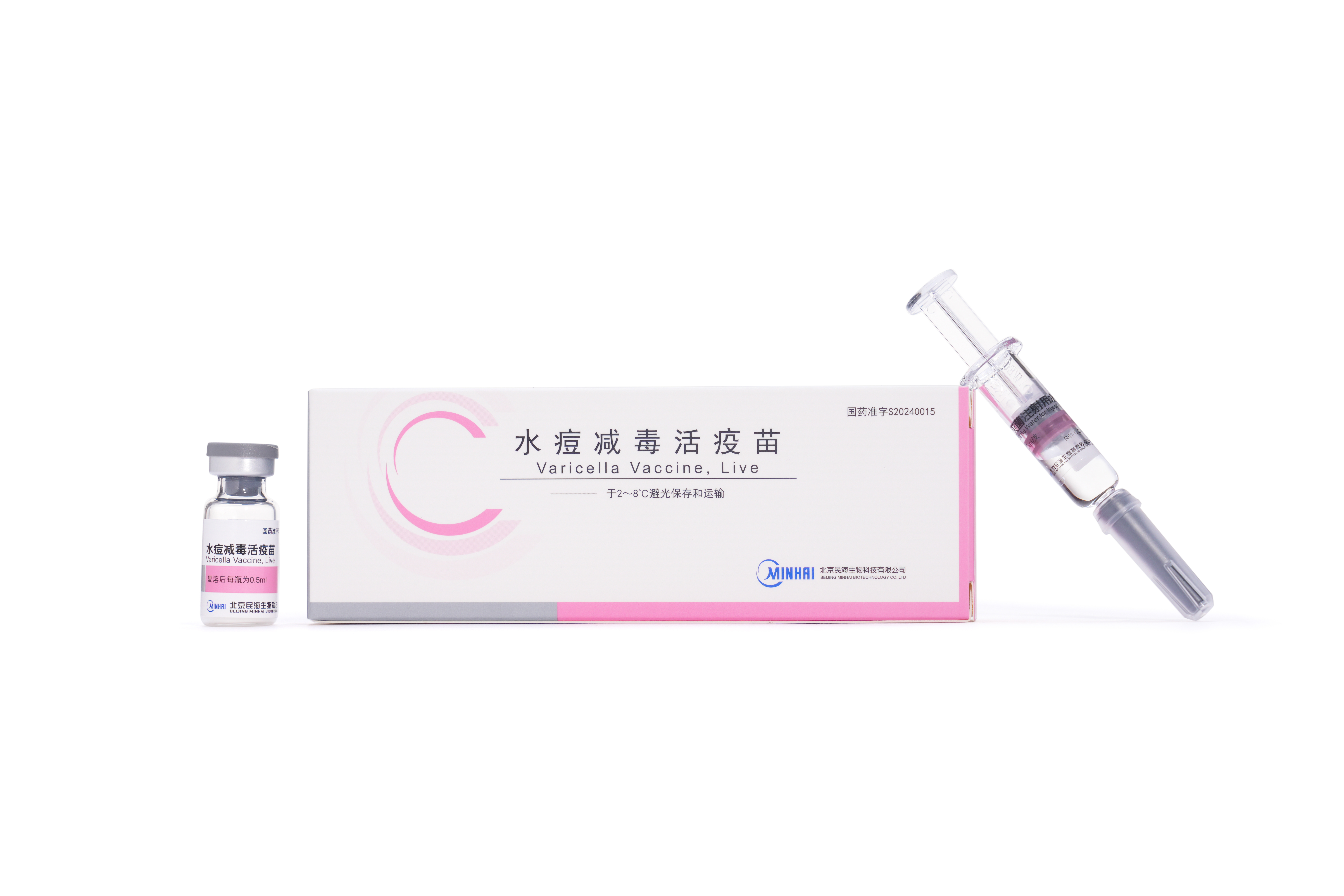
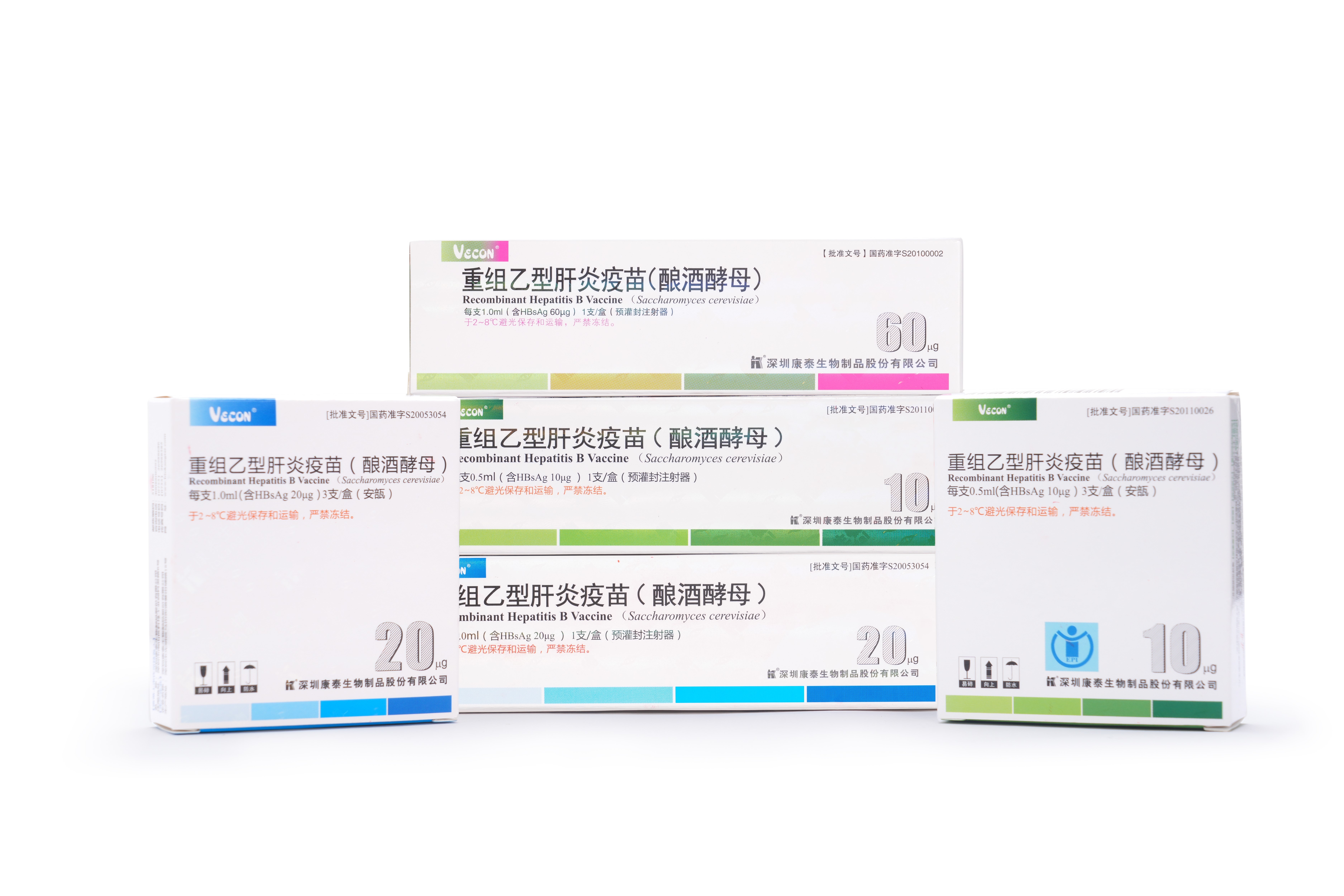
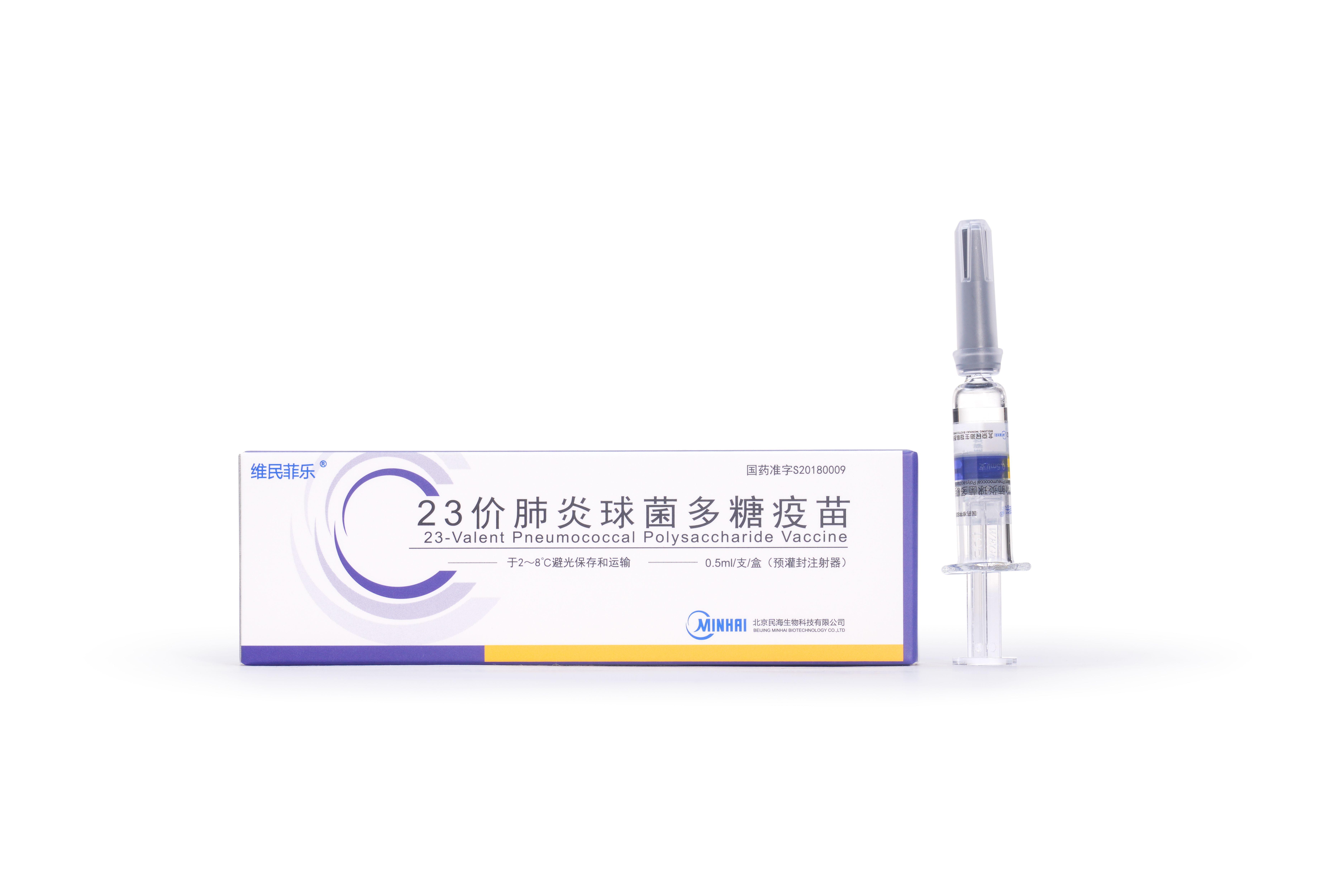
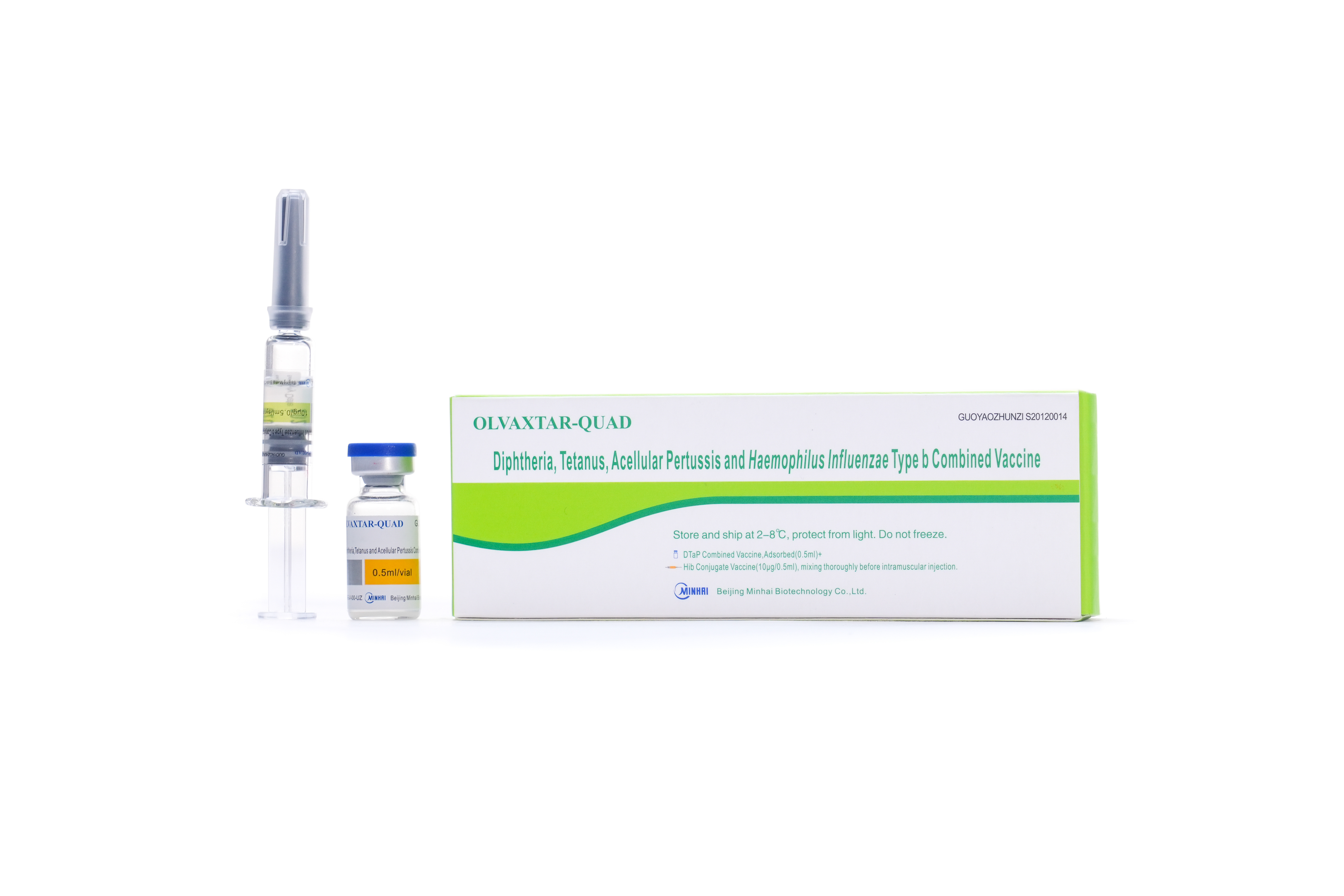
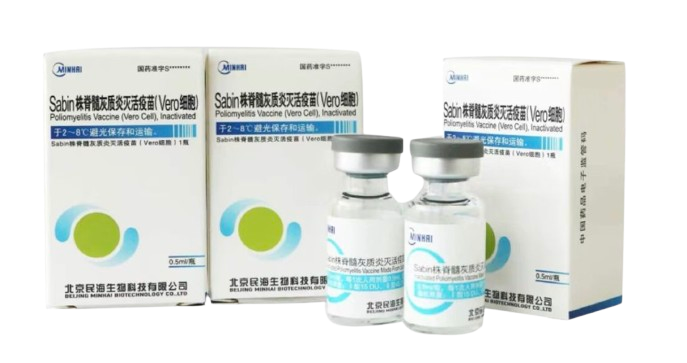
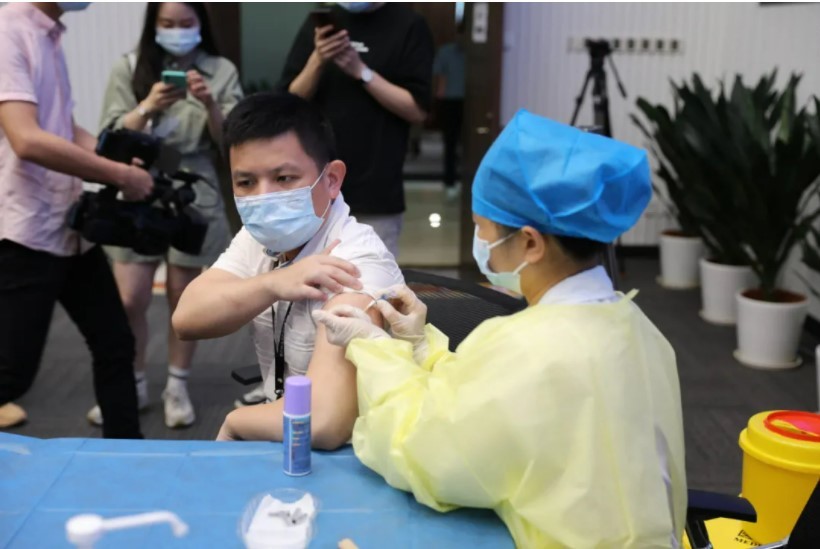
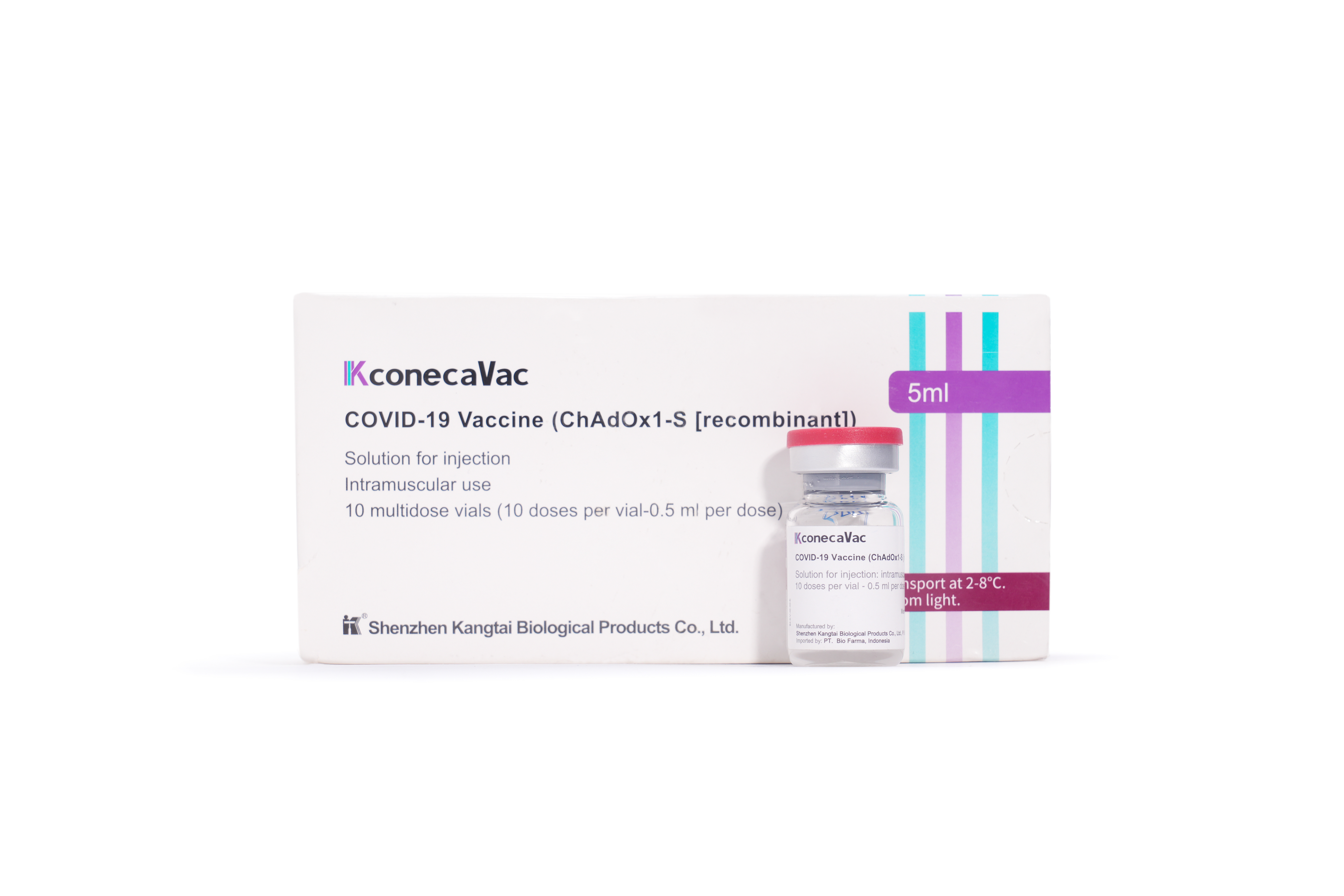
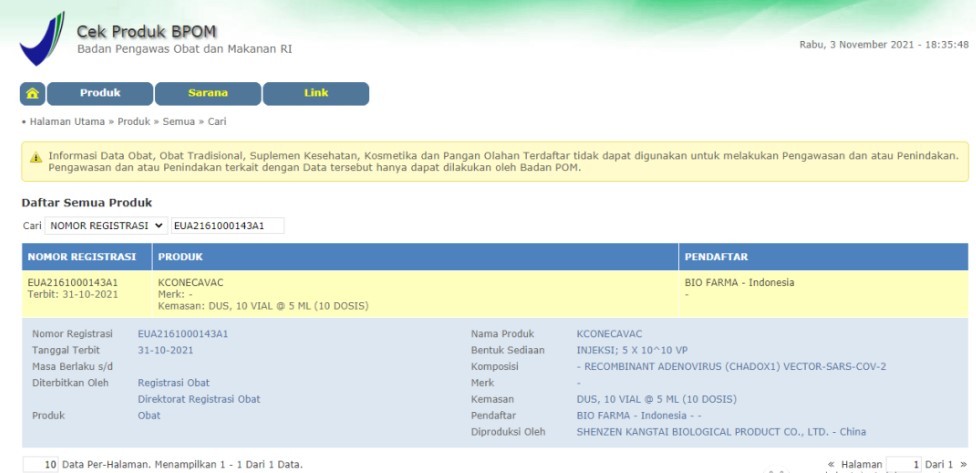
PRODUCTS
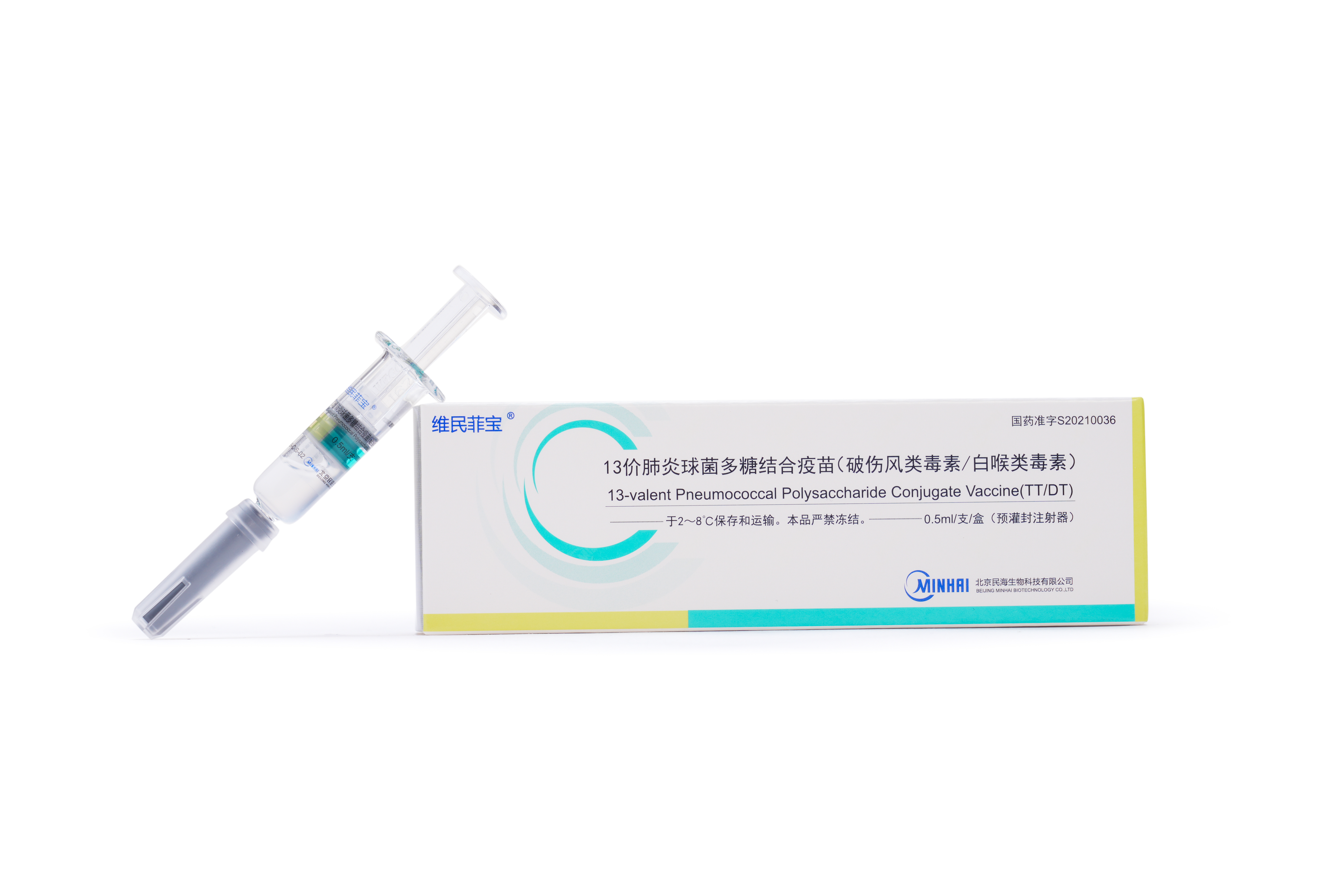
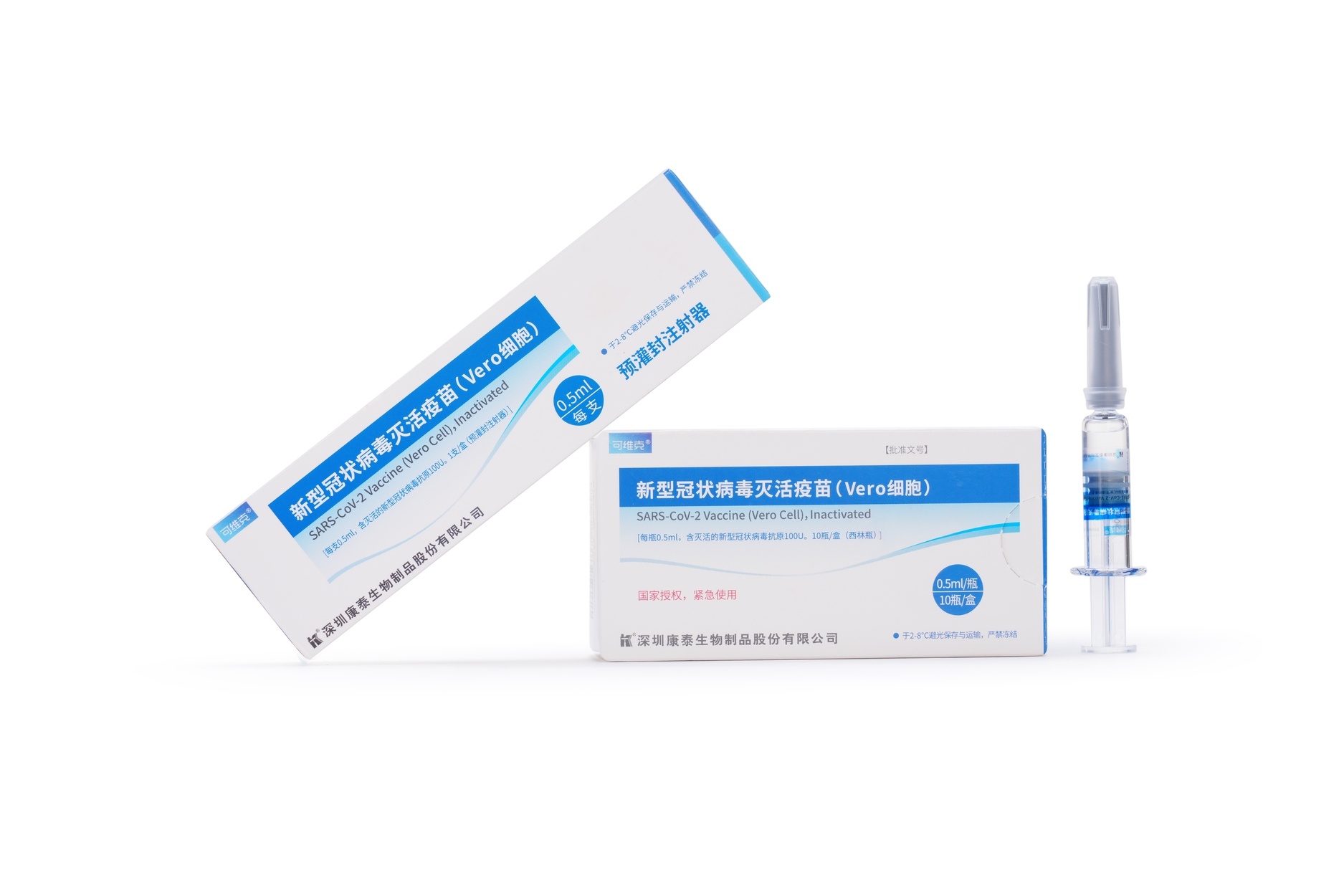
Launched Products
R&D Vaccine




ABOUT BIOKANGTAI
Leading innovative biopharmaceutical enterprise
Since its establishment in 1992, BioKangtai has undertaken a number of key scientific research projects and technology development tasks at the national, provincial, and municipal levels. It has processed core technologies such as polyvalent vaccines, genetic engineering vaccines, and other new technology R&D and industrialization core competitiveness.
Development History
+
Years
Production Base
m²
In Vaccine Research
+
Item
Research staff
+
Available vaccine varieties
+
view details
INTERNATIONAL BUSINESS DEVELOPMENT
Business development covers 6 regions and more than 20 countries.
view details
NEWS CENTER