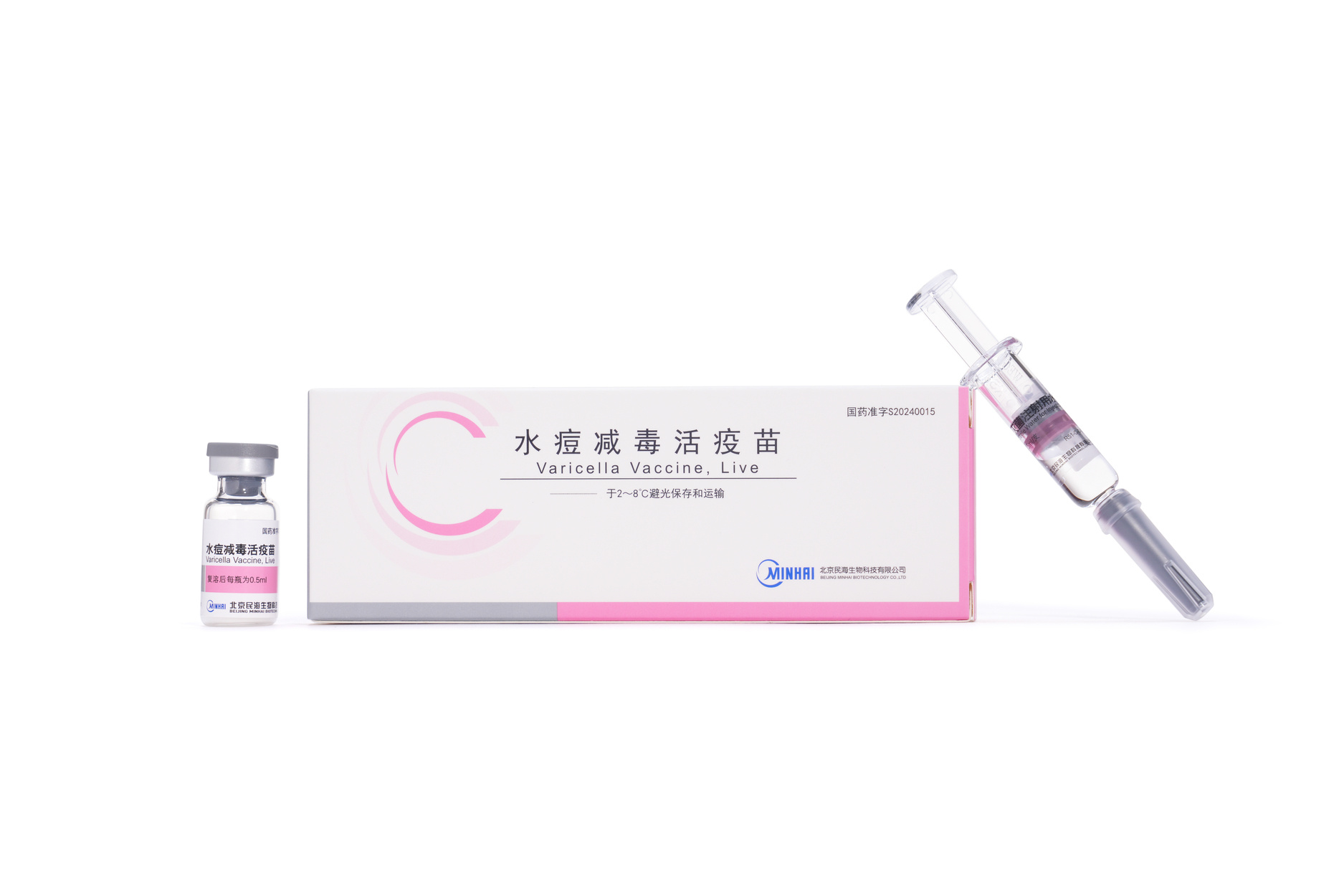
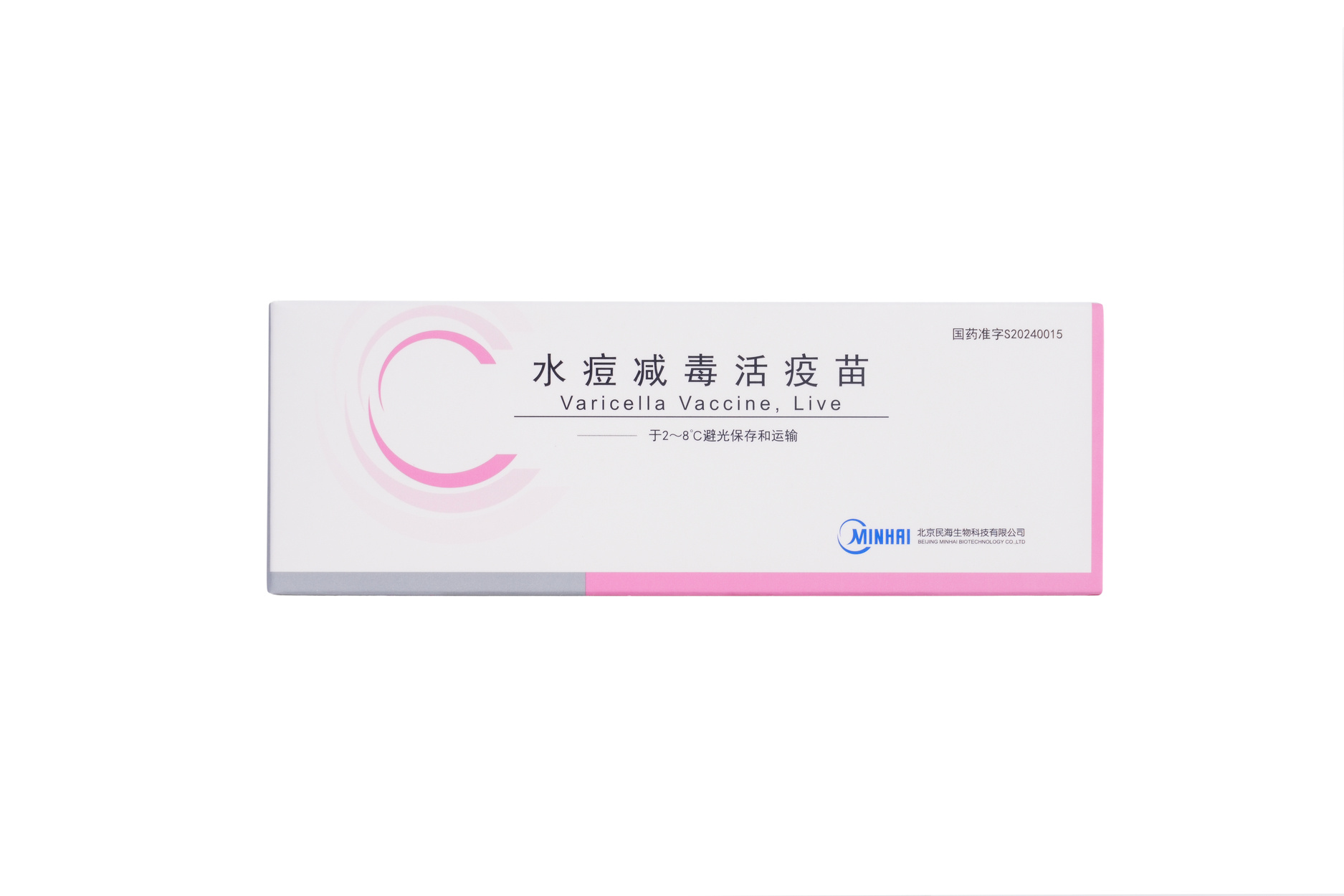
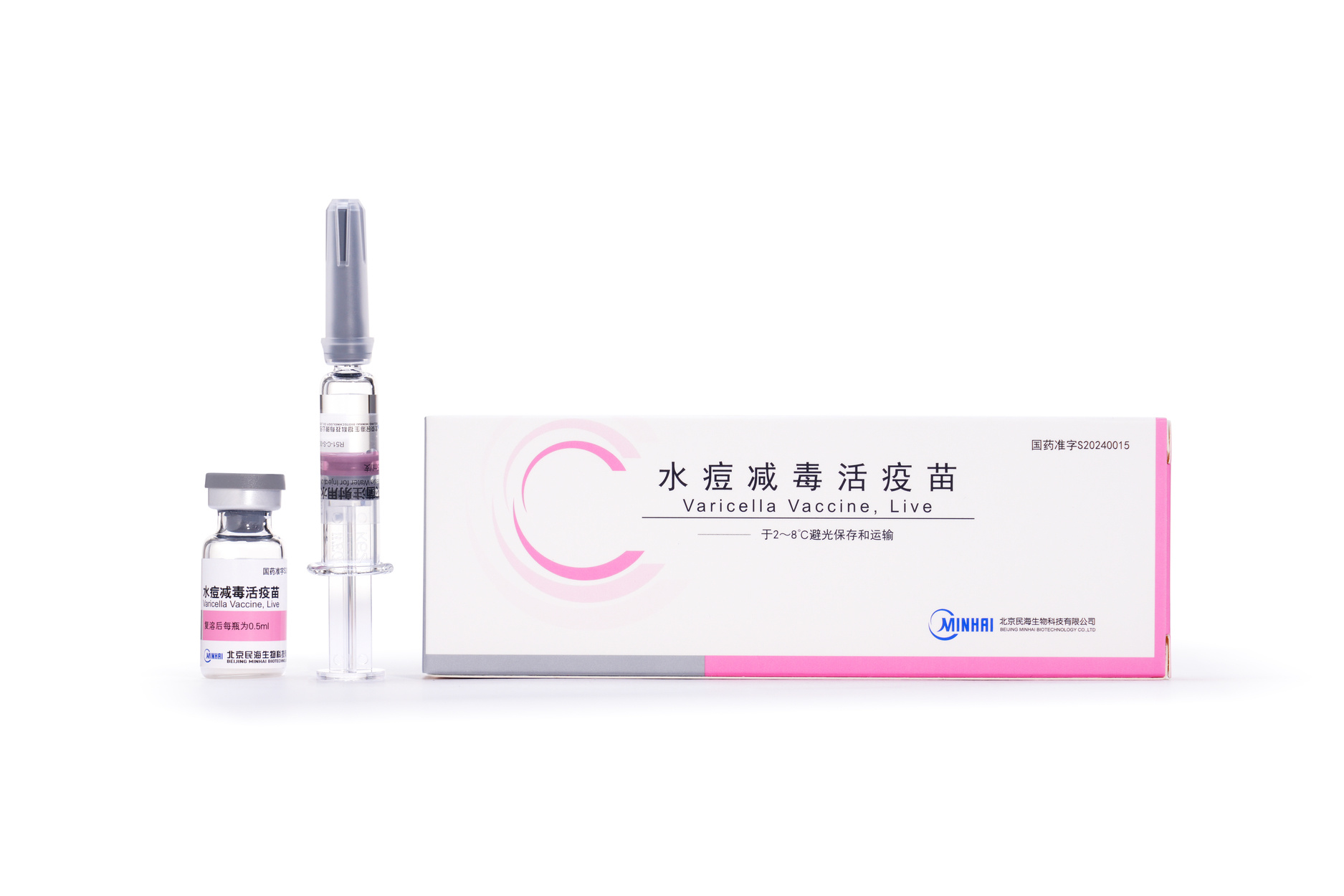
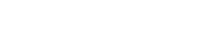Varicella Vaccine, Freeze-dried
Varicella Vaccine, Live
In 2024, it was approved by NMPA.
World-leading production process with significant advantages: The world‘s first use of ultrasound-crushing virus harvested in the outside cell factory, to avoid pollution, and be more efficient.
【Active constituent】 | Live varicella-zoster virus (Oka strain) |
【Strengths】 | 0.5 ml per vial after reconstitution. Each human dose is 0.5 ml, and the live varicella-zoster virus is not less than 3.3 lgPFU. |
【Eligibles】 | Varicella susceptible persons over 1 year old. |
【Shelf Life】 | 24 months. |