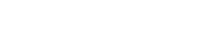ABOUT US
History Of Biokangtai
2023
HDCV Rabies vaccine obtained the approval
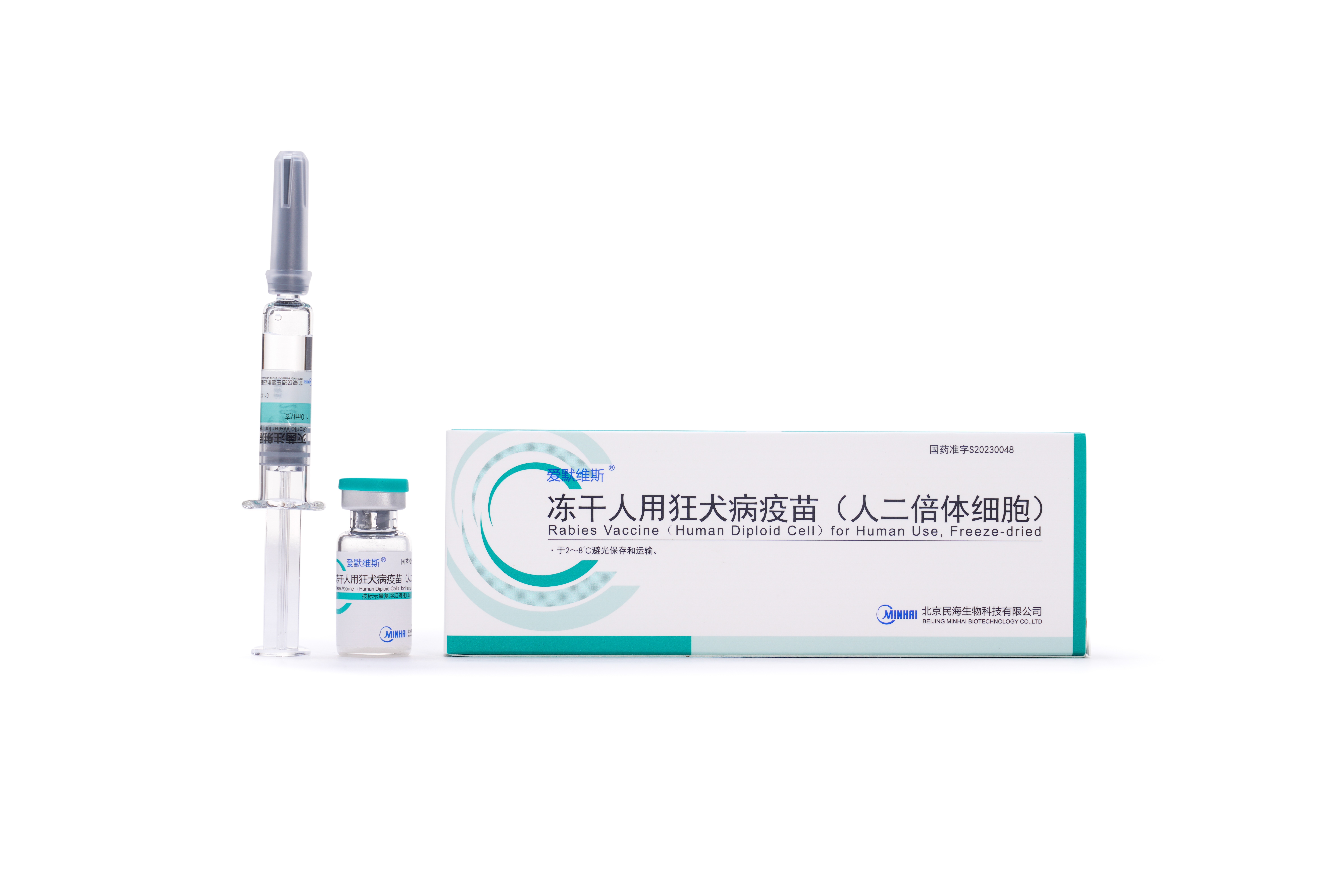
2021
SARS-CoV-2 Vaccine, inactivated, EUA, China;
AZD1222,EUA ,Indonesia;
PCV13 obtained the approval
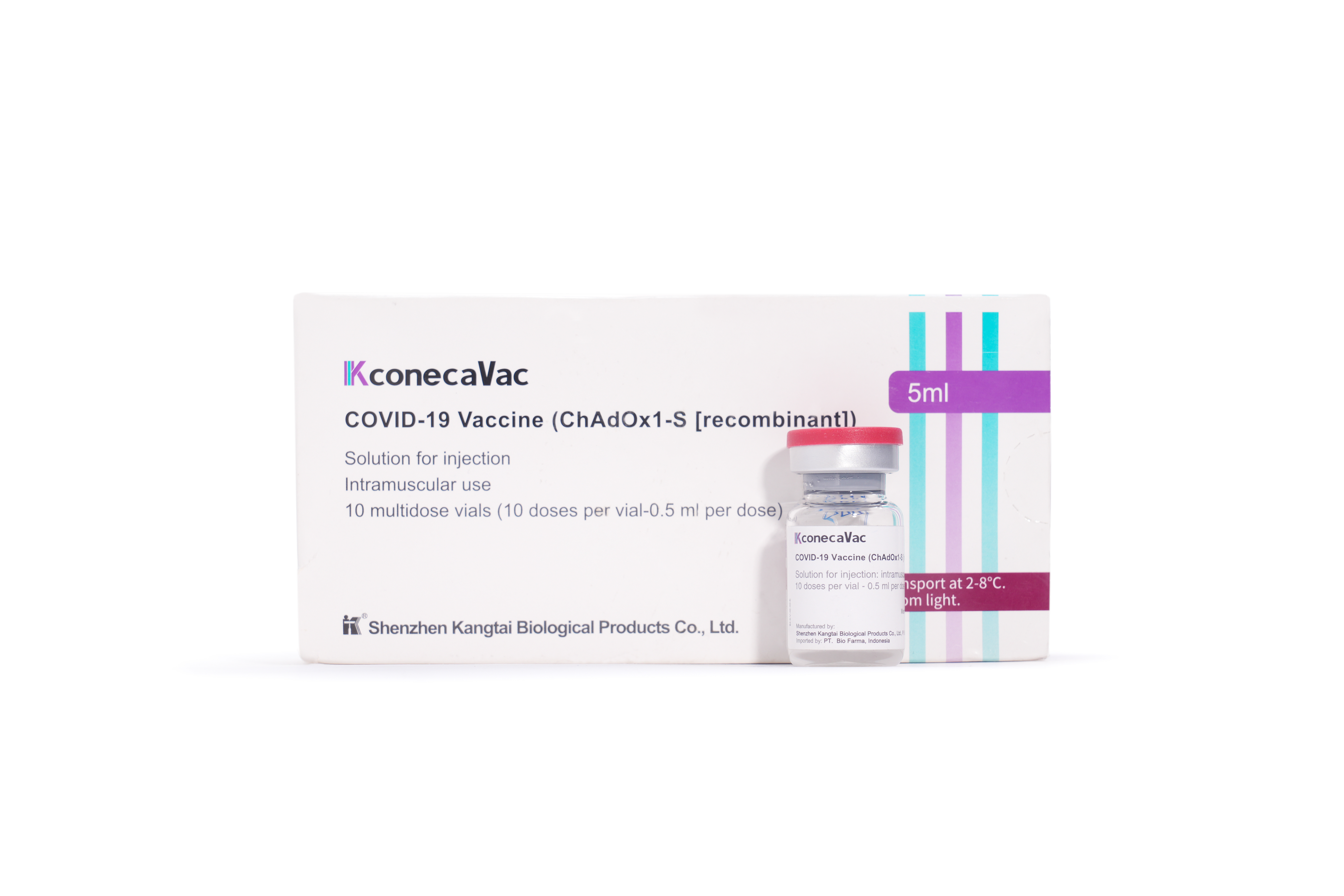
2020
DTaP Vaccine obtained the approval
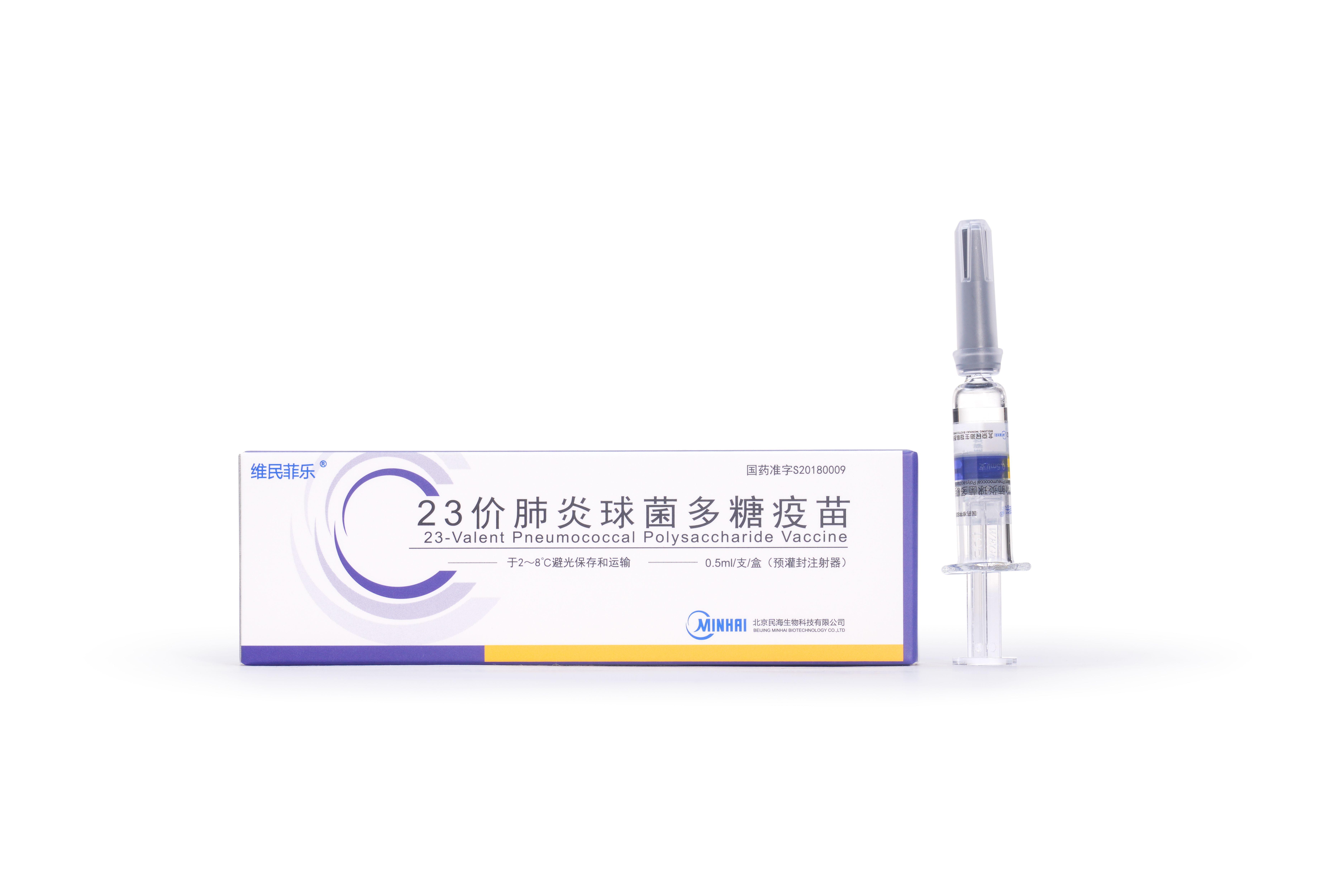
2019
23-Valent Pneumonia Vaccine received lot release and was officially launched into market.
2017
Listed on ChiNext board of Shenzhen Stock Exchange in February 2017, stock code 30060.
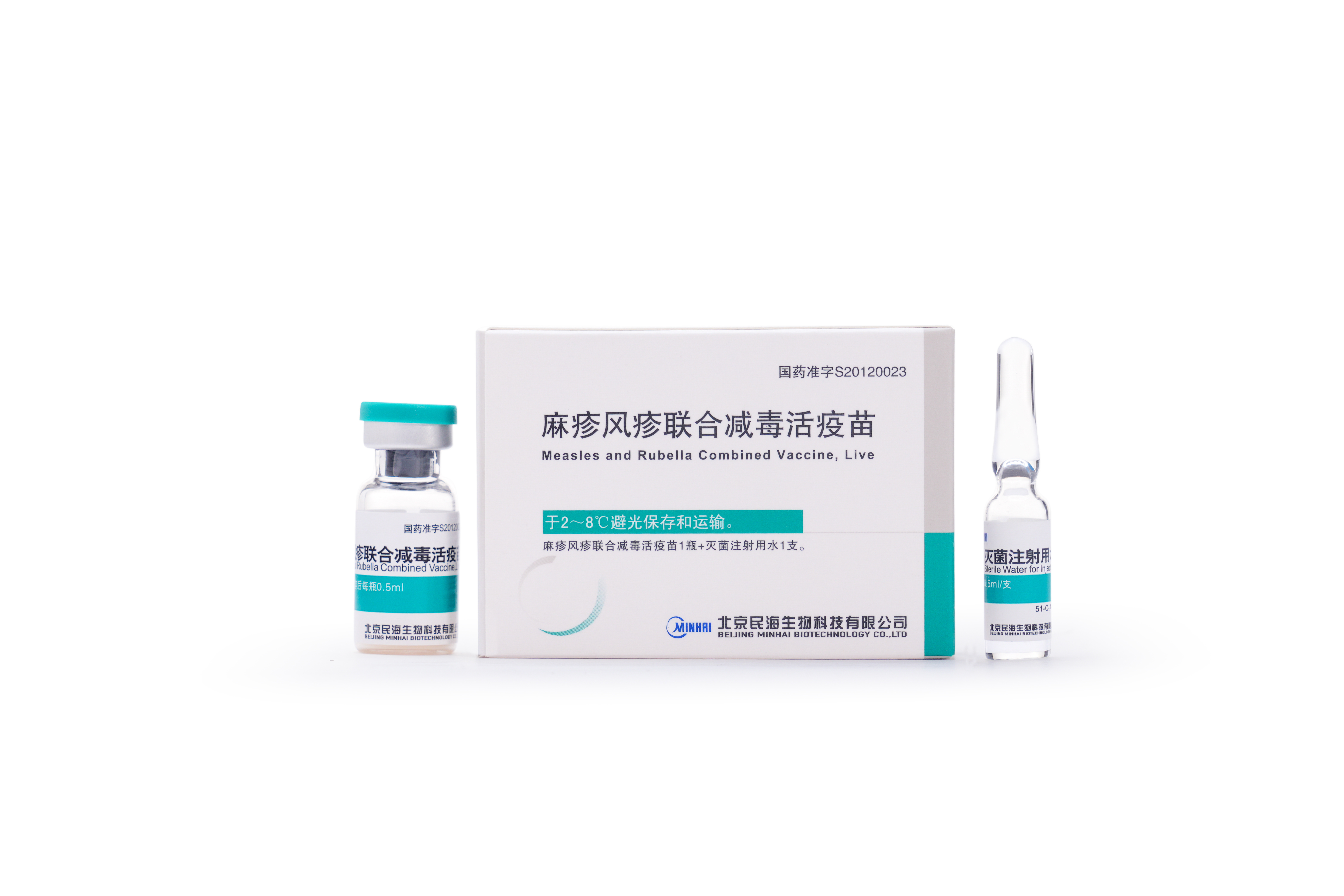
2013
MR vaccine, DTaP-Hib combined vaccine passed GMP Certification and obtained the approval
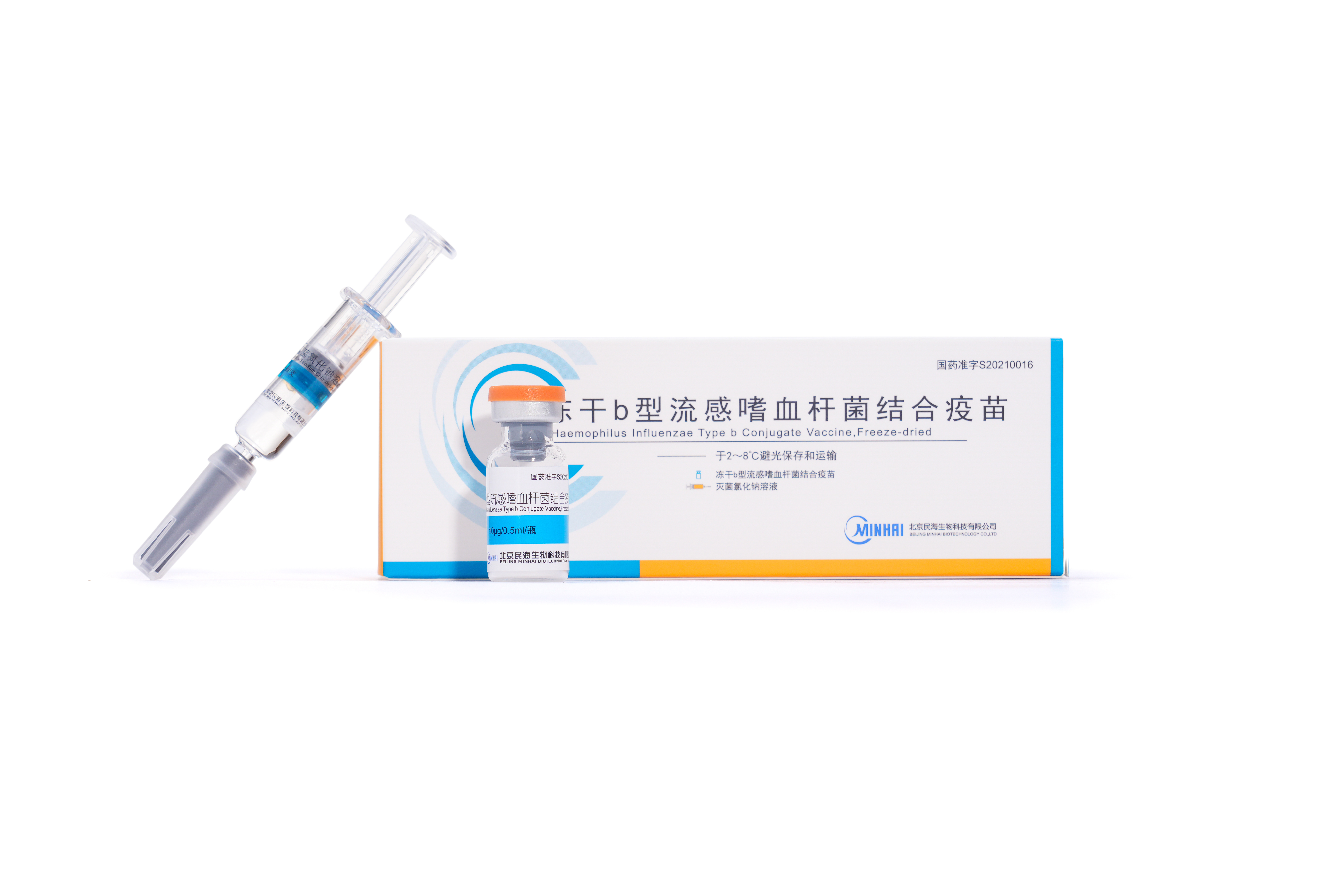
2012
Haemophilus influenzae type b conjugate vaccine passed GMP certification.

2008
In a strategic reorganization,BioMinhai became a wholly-owned subsidiary of Biokangtai
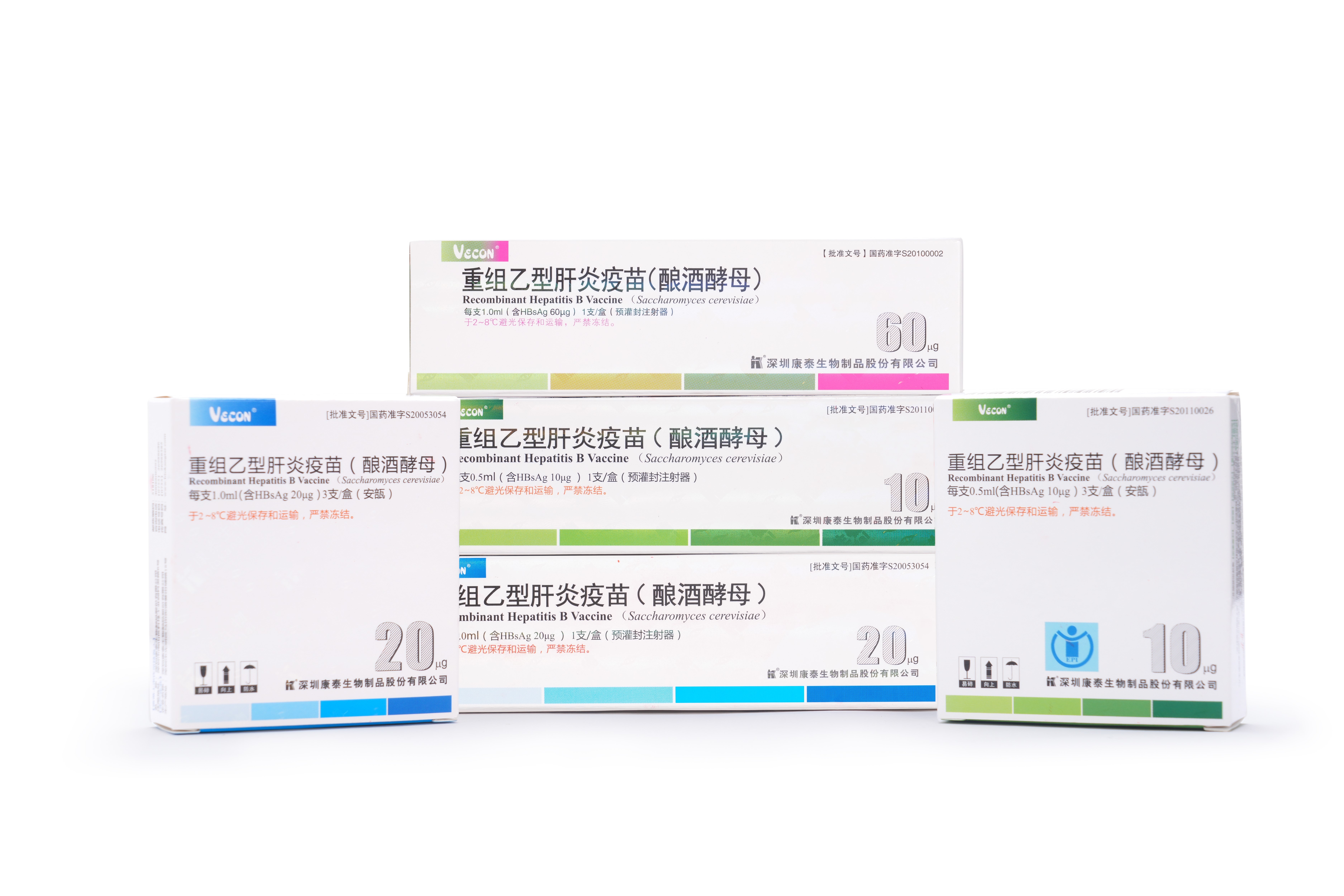
1995
5 μg/0.5ml/standard hepatitis B vaccine meets Merck's quality standards. Obtaining the Ministry of Health trial production approval number.
1998: Obtained the official approval number from the State Food and Drug Administration

1992
BioKangtai established


2023
2021
2020
2019
2017
2013
2012
2008
1995
1992
Copyright © 1992-2024 Shenzhen Kangtai Biological Products Co., Ltd.