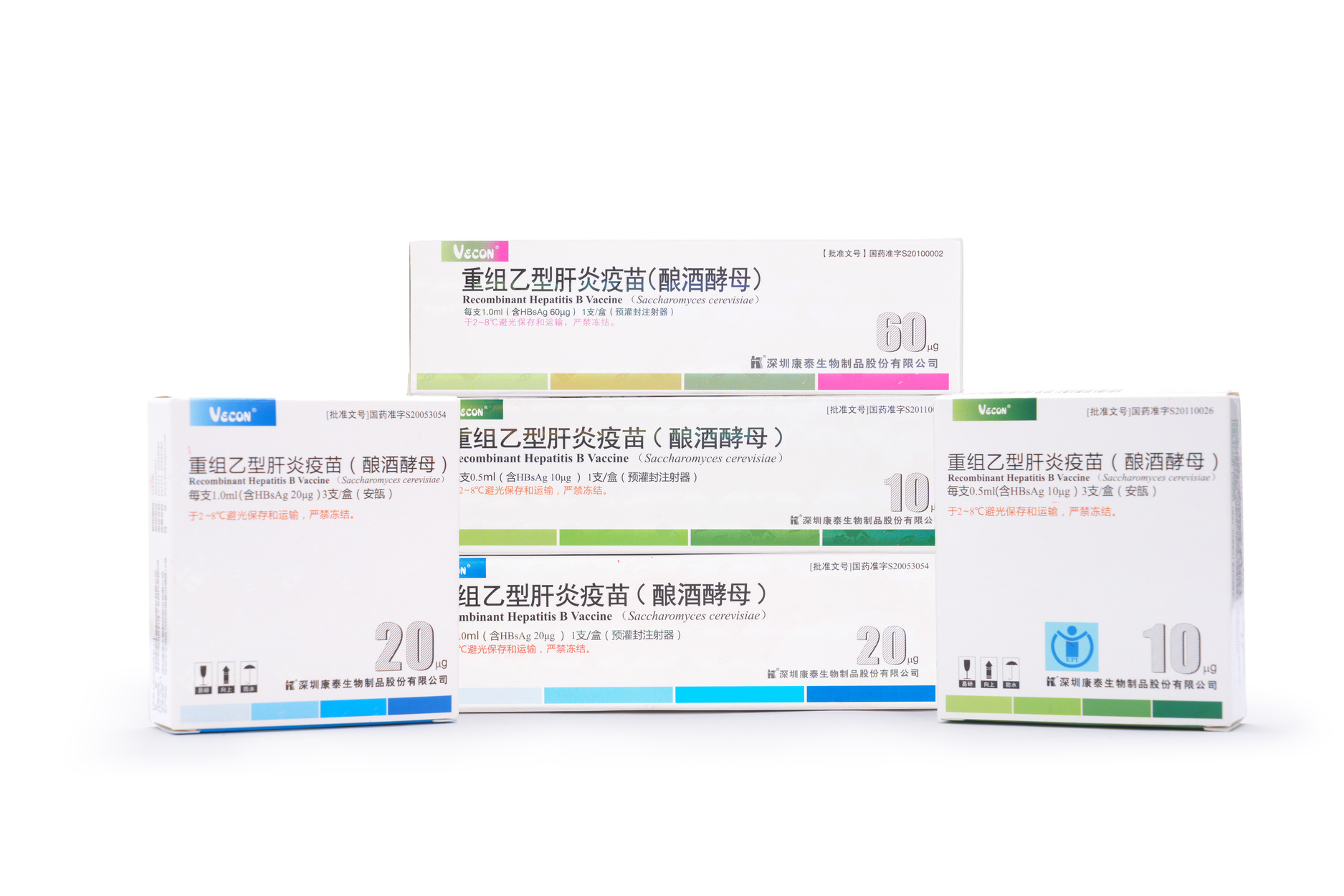
Hepatitis B Vaccine Family
Hepatitis B Vaccine Family
In 2010, Developed 60μg Recombinant Hepatitis B vaccine (Saccharomyces cerevisiae);
In 2011, Reescalation of hepatitis B vaccine dose: 10μg in children and 20μg in adults;
In 2023, Kangtai Recombinant Hepatitis B vaccine (Saccharomyces cerevisiae) produced more than 1.4 billion doses.
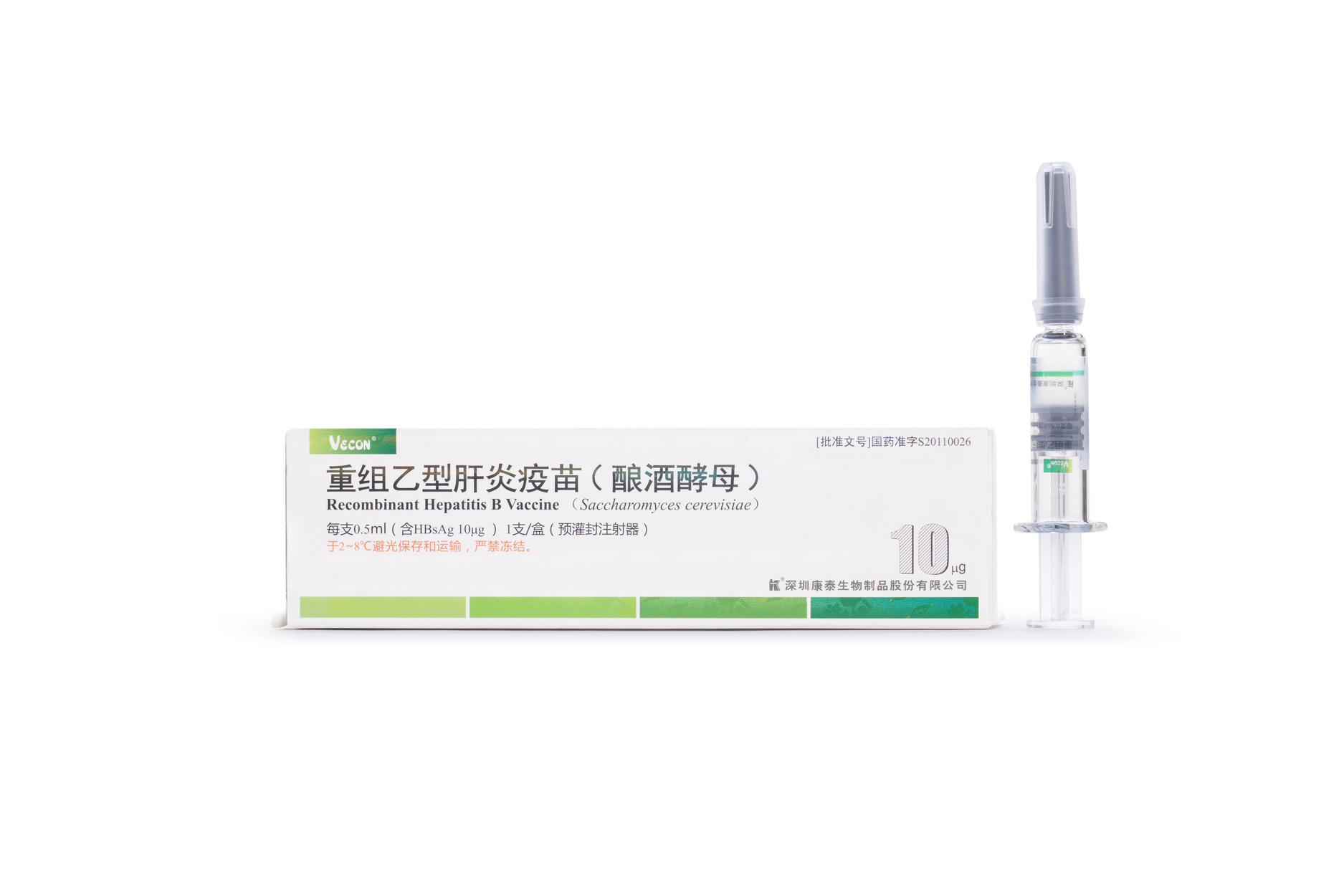
10μg Recombinant Hepatitis B Vaccine (Saccharomyces cerevisiae)
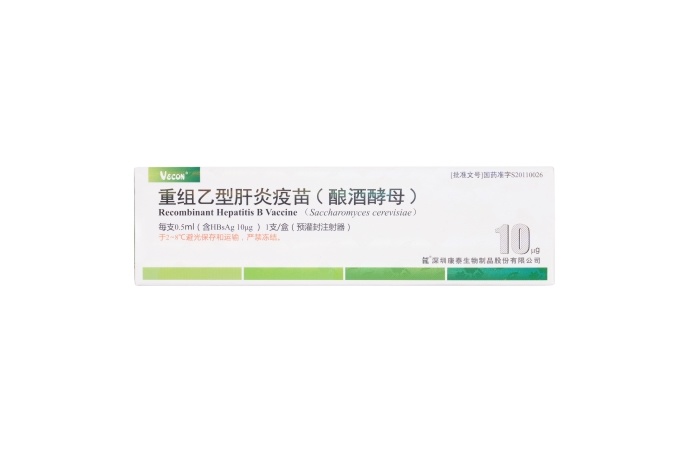
【Active constituent】 | Hepatitis B surface antigen. |
【Strengths】 | 0.5ml per container. 0.5 ml per single human dose containing 10 μg of HBsAg. |
【Eligibles】 | Hepatitis B susceptible populations aged below 16 years, especially newborns whose mother has positive HBsAg and/or HBeAg result. |
【Shelf Life】 | 36 Months |
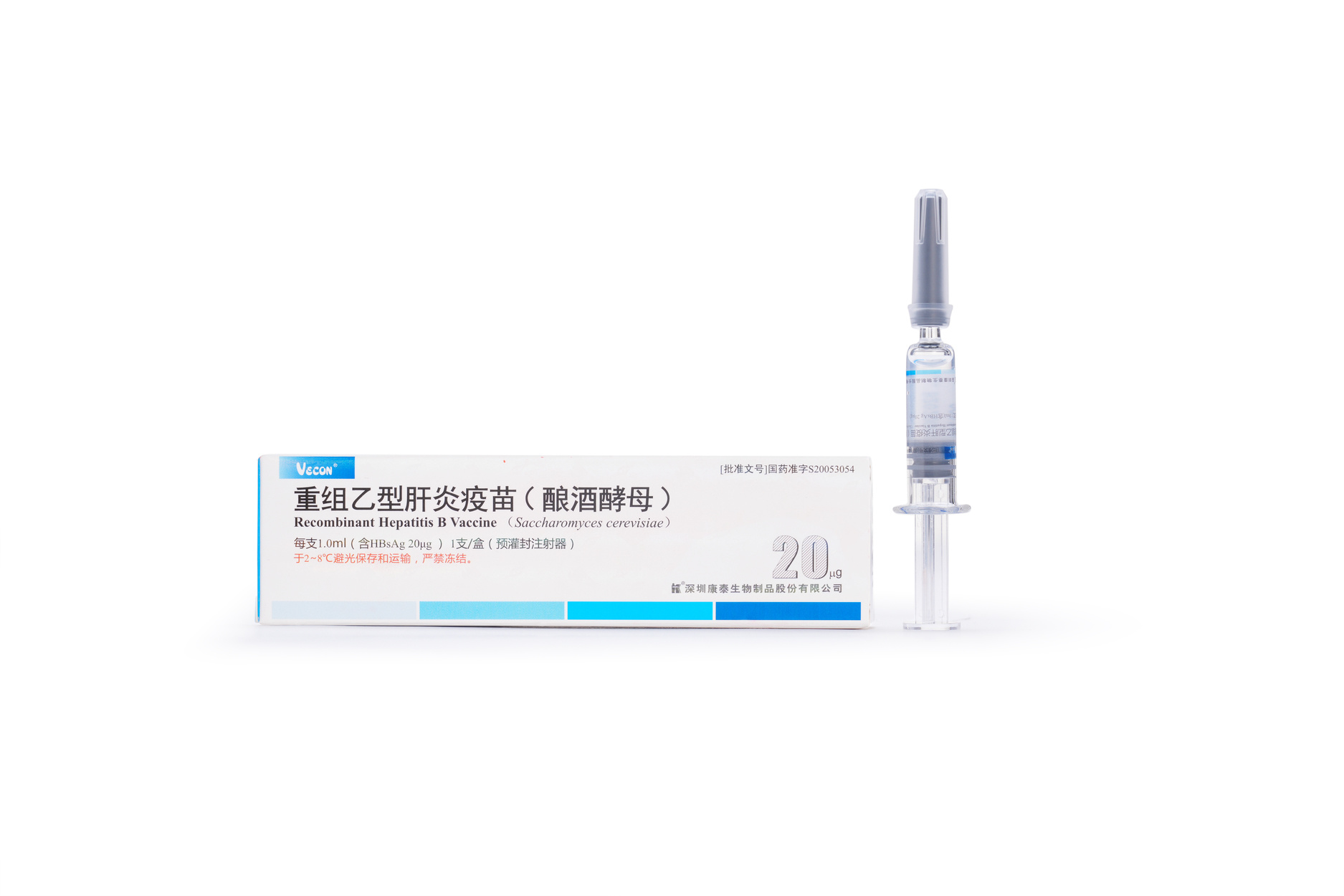
20μg Recombinant Hepatitis B Vaccine (Saccharomyces cerevisiae)
National "12th Five-Year Plan" Major Science and Technology Special Projects;
Certificate of National Key New Products.

【Active constituent】 | Hepatitis B surface antigen. |
【Strengths】 | 1.0ml per container.1.0 ml per single human dose containing 20 μg of HBsAg. |
【Eligibles】 | Hepatitis B susceptible populations aged 16 years and above. |
【Shelf Life】 | 36 Months |
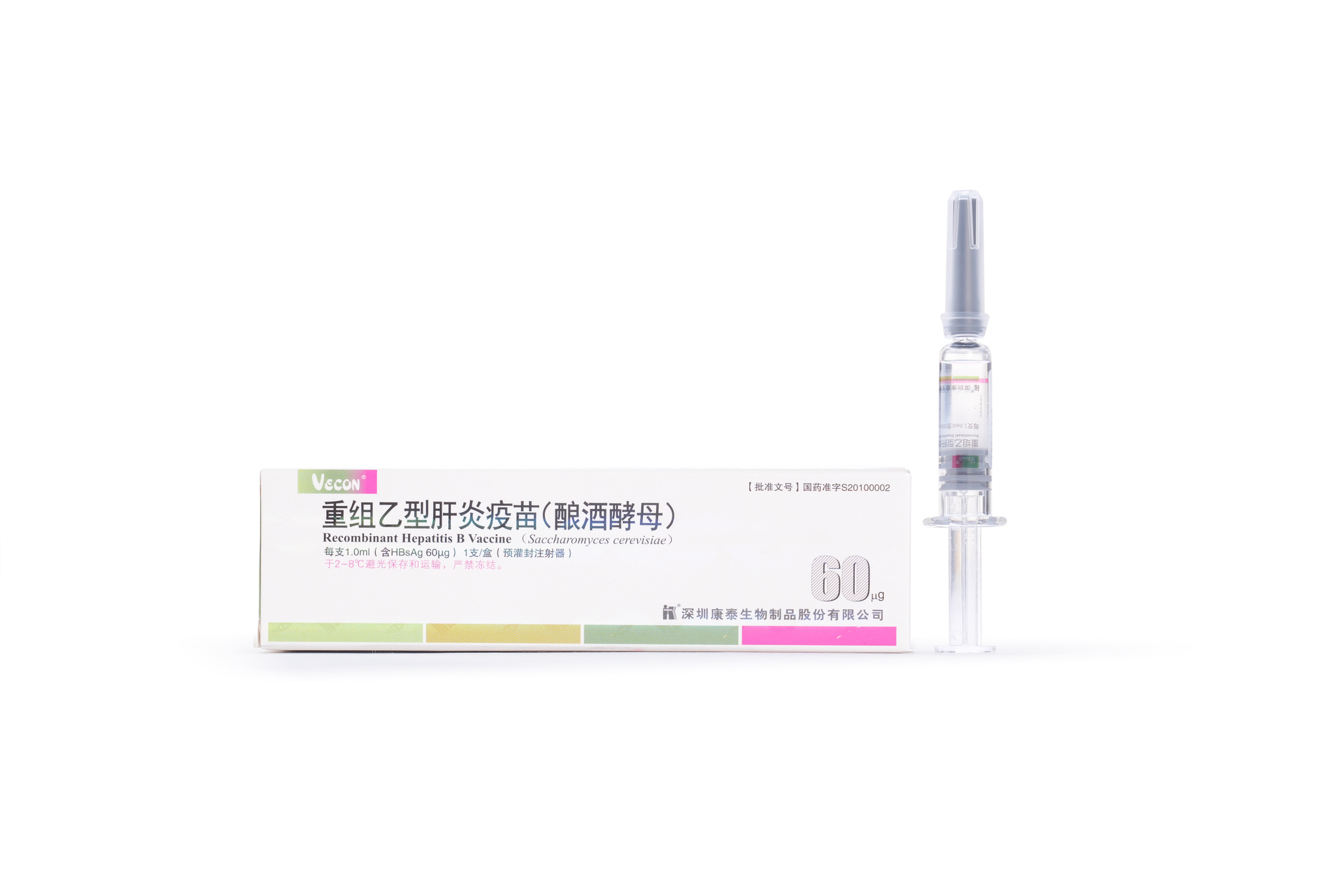
60μg Recombinant Hepatitis B Vaccine (Saccharomyces cerevisiae)
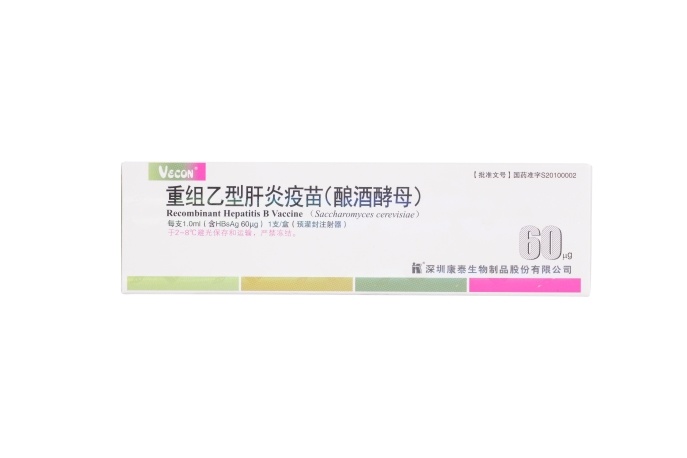
【Active constituent】 | Hepatitis B surface antigen. |
【Strengths】 | 1.0ml per container.1.0 ml per single human dose containing 60 μg of HBsAg. |
【Eligibles】 | Hepatitis B susceptible populations aged above 16 years and who don’t respond to routine immunization with Hepatitis B vaccine. |
【Shelf Life】 | 36 Months |