BioKangtai's Adenovirus Vector COVID-19 Vaccine Obtained EUA in Indonesia
Date:2021.11.03
类型: 行业动态
分享:


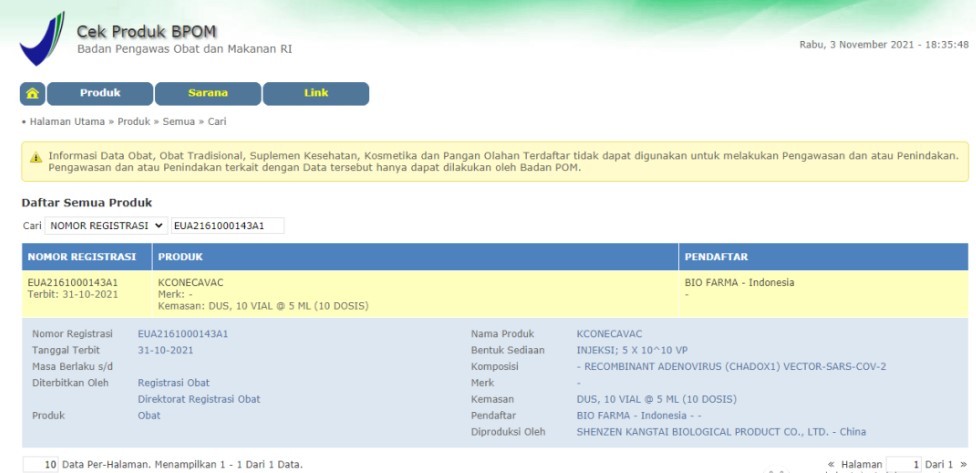
SHENZHEN, China, Nov. 3, 2021 /PRNewswire/ -- As reported by Indonesian National Agency of Drug and Food Control (BPOM), BPOM grants the Emergency Use Authorization (EUA) for COVID-19 Vaccine (ChAdOx1-S[recombinant])/KconecaVac manufactured by Shenzhen Kangtai Biological Products Co., Ltd.. It is understood that this is China's second adenovirus vector COVID-19 vaccine approved for emergency use overseas.
BPOM source:
http://cekbpom.pom.go.id//home/produk/ia50q76n48d7ou9ibfgcn3rik7/all/row/10/page/1/order/4/DESC/search/0/EUA
SOURCE Shenzhen Kangtai Biological Products Co., Ltd.
Company Codes: Shenzhen: 300601