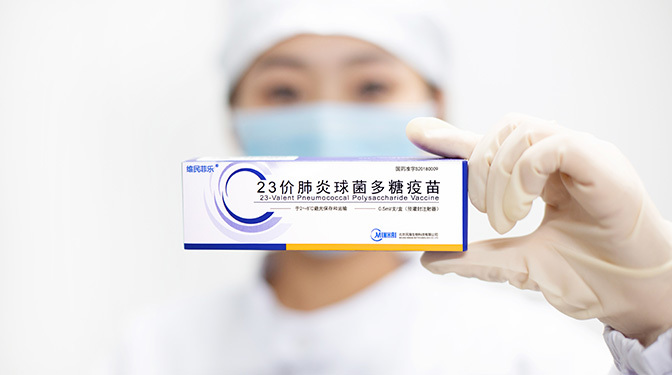
23-Valent Pneumococcal Polysaccharide Vaccine
23-Valent Pneumococcal Polysaccharide Vaccine
In 2019, it was approved by NMPA, China.
The key project of the science and technique ministry of China.
【Active constituent】 | Pneumonia serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 20, 22F, 23F, 33F |
【Strengths】 | 0.5ml per vial. 0.5 ml per single human dose containing 23 serotypes of purified peneumococcal capsular polusaccharde respectively 25 μg. |
【Eligibles】 | People aged 2 years and above, especially for people who suffer from chronic diseases, who suffer from immunological functions, who are HIV-infected, who with cerebrospinal fluid leak, who live or work in a high-risk environment with pneumonia, and who are the elders. |
【Shelf Life】 | 24 months |